Optimization and Scale-Up of a Suzuki−Miyaura Coupling Reaction: Development of an Efficient Palladium Removal Technique | Organic Process Research & Development
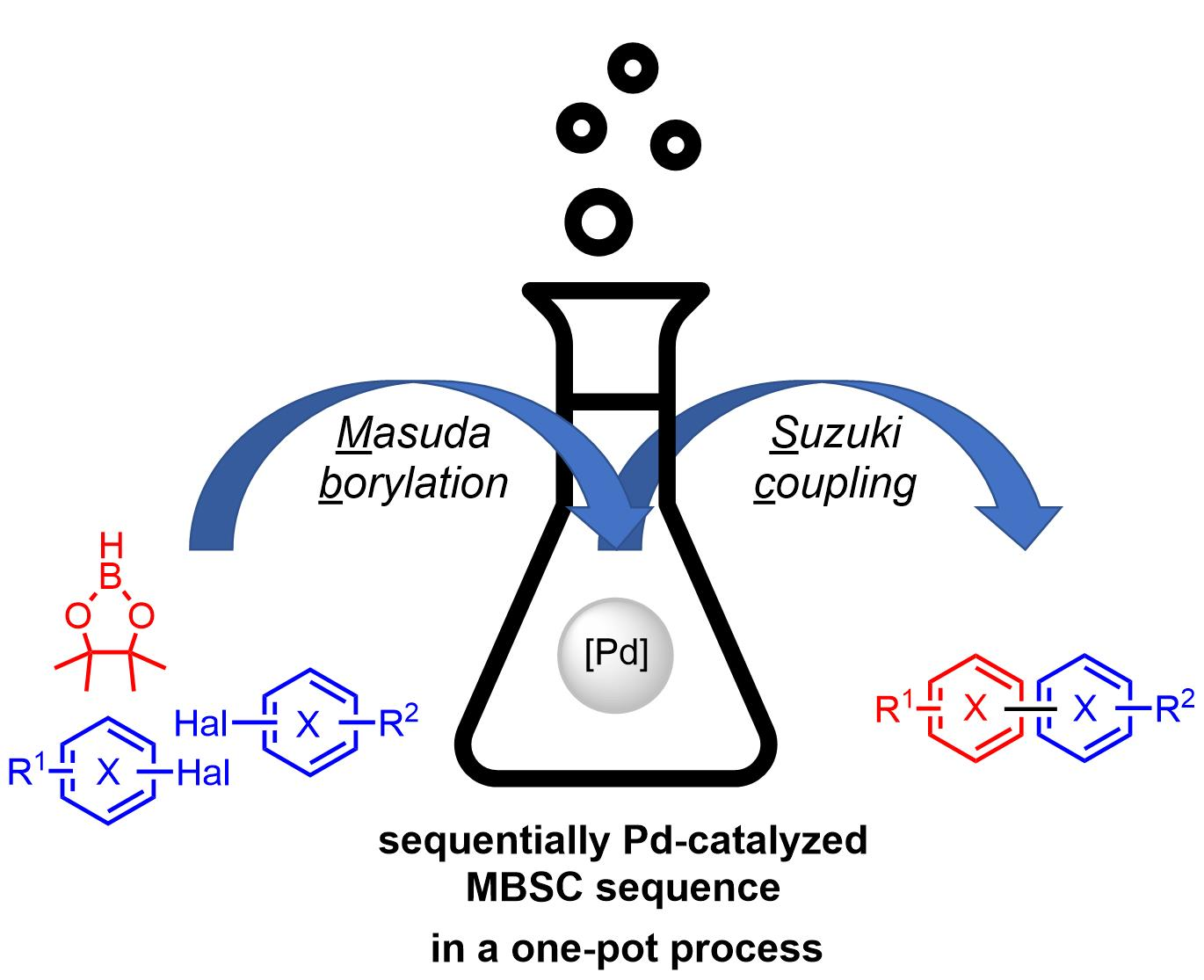
Catalysts | Free Full-Text | Masuda Borylation–Suzuki Coupling (MBSC) Sequence: A One-Pot Process to Access Complex (hetero)Biaryls

Palladium-Catalyzed Double-Suzuki–Miyaura Reactions Using Cyclic Dibenziodoniums: Synthesis of o-Tetraaryls | The Journal of Organic Chemistry
Organics | Free Full-Text | Recent Applications of Pd-Catalyzed Suzuki–Miyaura and Buchwald–Hartwig Couplings in Pharmaceutical Process Chemistry

Suzuki−Miyaura Cross-Coupling Reactions of Primary Alkyltrifluoroborates with Aryl Chlorides | The Journal of Organic Chemistry

Suzuki-Miyaura Cross-Coupling Reaction and Potential Applications: Kostas, Ioannis D: 9783038425564: Amazon.com: Books

Pre-transmetalation intermediates in the Suzuki-Miyaura reaction revealed: The missing link | Science
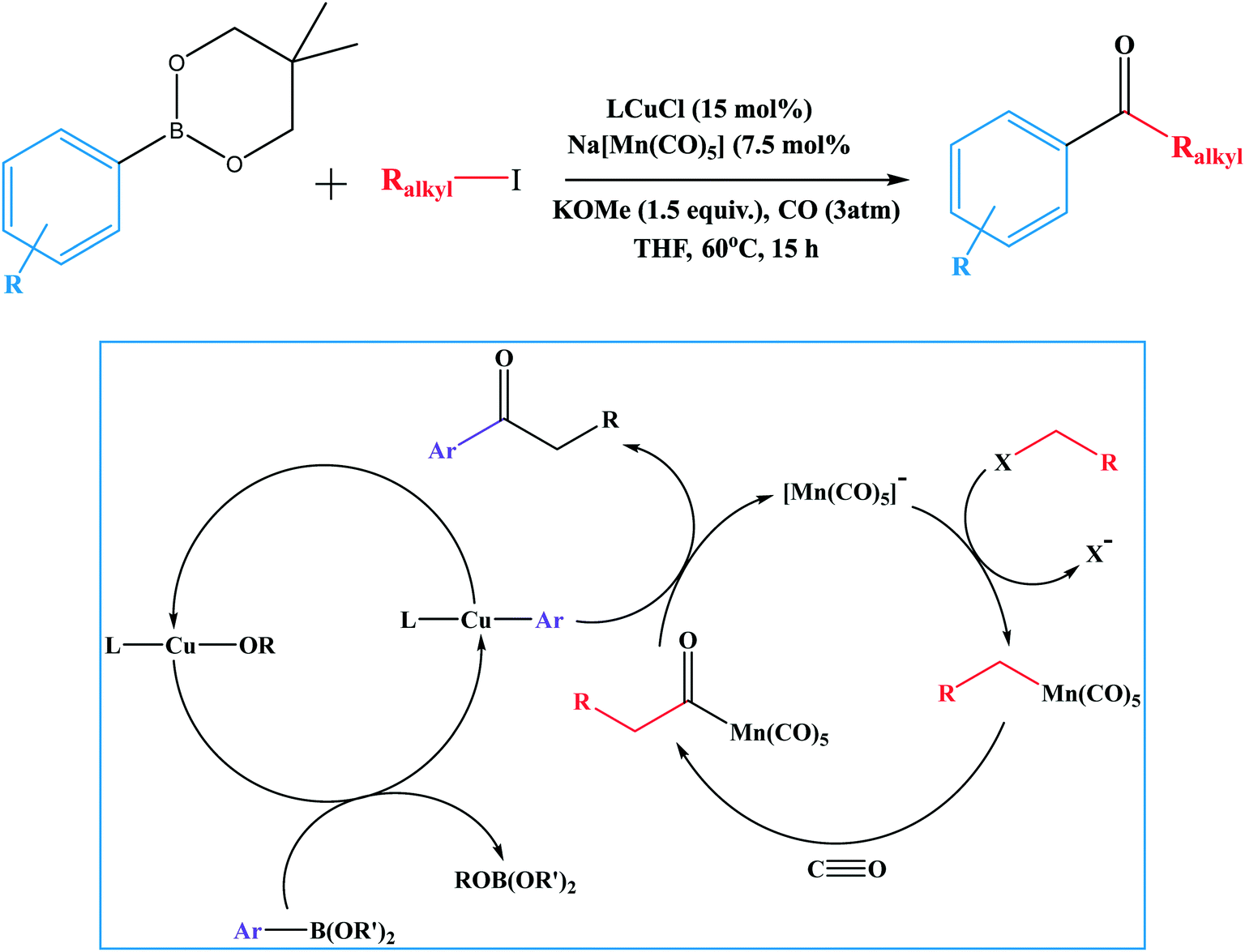
Suzuki–Miyaura cross coupling reaction: recent advancements in catalysis and organic synthesis - Catalysis Science & Technology (RSC Publishing) DOI:10.1039/D0CY02059A

Ligand-Free Suzuki–Miyaura Coupling Reactions Using an Inexpensive Aqueous Palladium Source: A Synthetic and Computational Exercise for the Undergraduate Organic Chemistry Laboratory | Journal of Chemical Education

Scheme 1. Mechanism of the homogeneous Suzuki-Miyaura reaction. Scheme... | Download Scientific Diagram

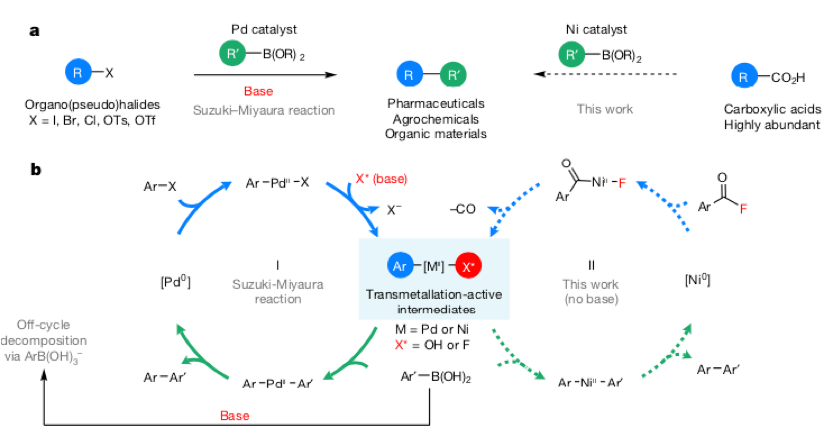
Synthesis of Biaryls via Decarbonylative Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling of Carboxylic Acids - ScienceDirect

Palladium catalyzed asymmetric Suzuki–Miyaura coupling reactions to axially chiral biaryl compounds: Chiral ligands and recent advances - ScienceDirect
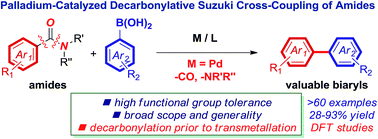
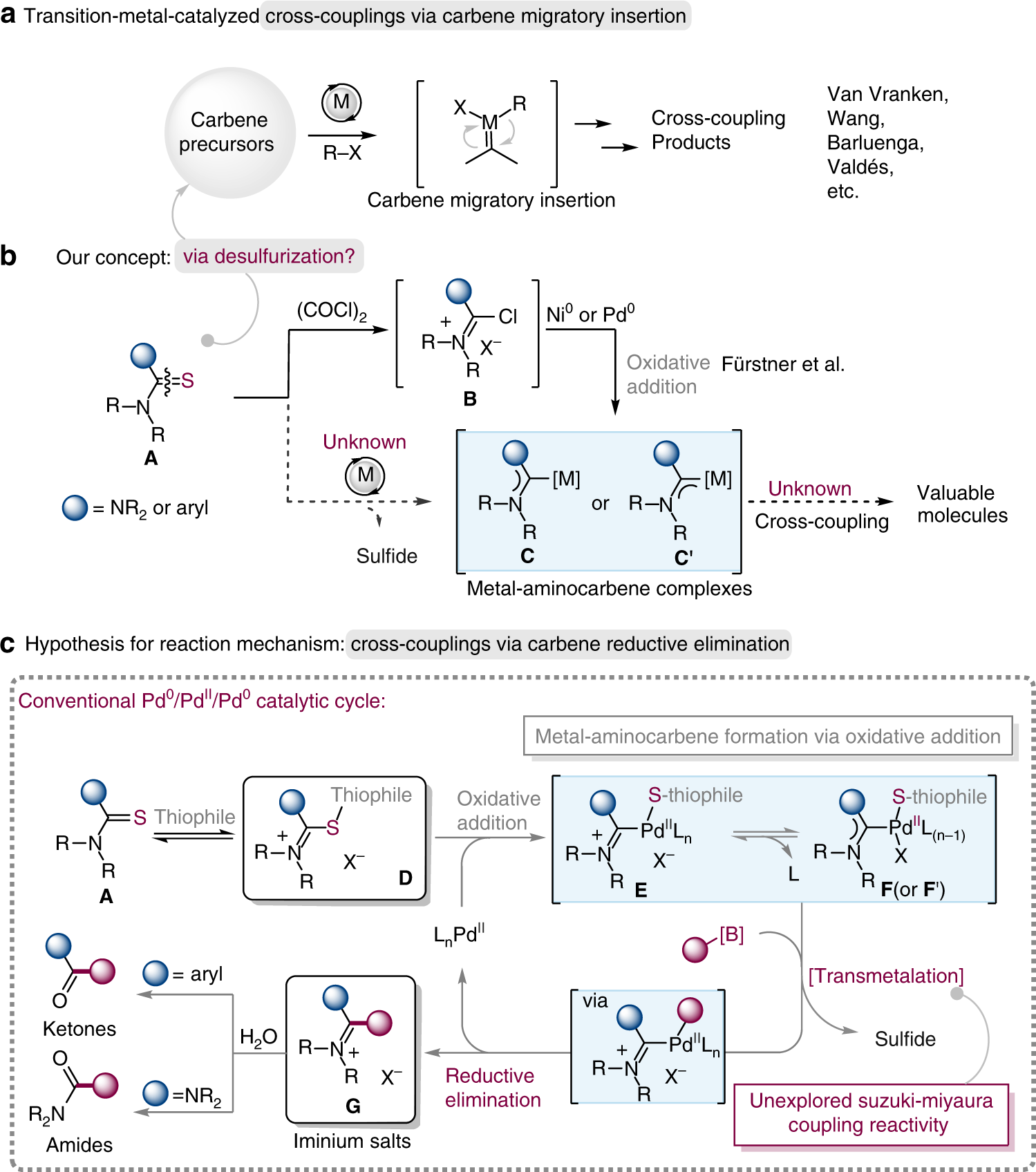
Palladium-catalyzed decarbonylative Suzuki–Miyaura cross-coupling of amides by carbon–nitrogen bond activation - Chemical Science (RSC Publishing)
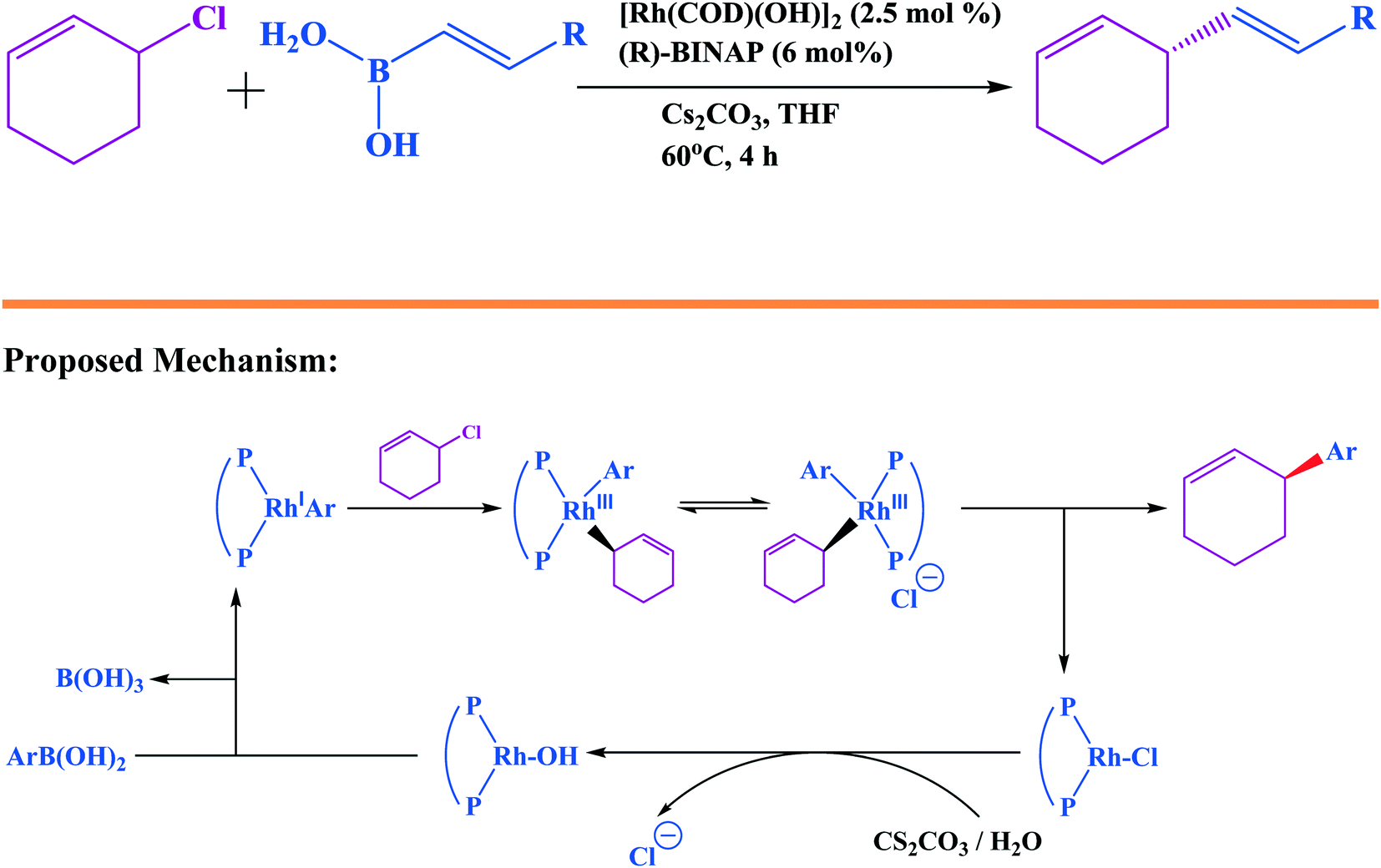
Suzuki–Miyaura cross coupling reaction: recent advancements in catalysis and organic synthesis - Catalysis Science & Technology (RSC Publishing) DOI:10.1039/D0CY02059A

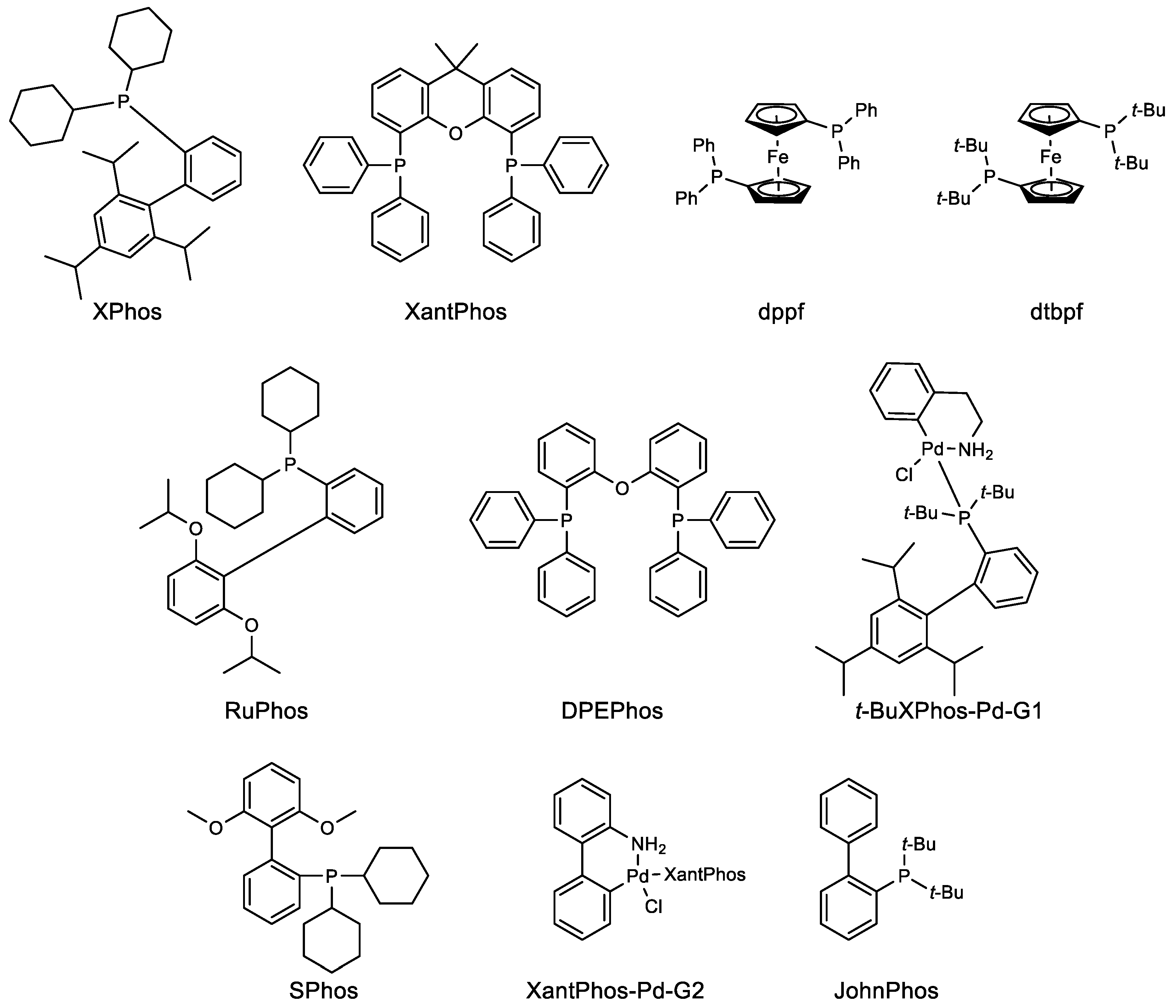
Catalysts for Suzuki−Miyaura Coupling Processes: Scope and Studies of the Effect of Ligand Structure | Journal of the American Chemical Society

Base-Free Suzuki–Miyaura Coupling Reaction Using Palladium(II) Supported Catalyst in Water | Catalysis Letters
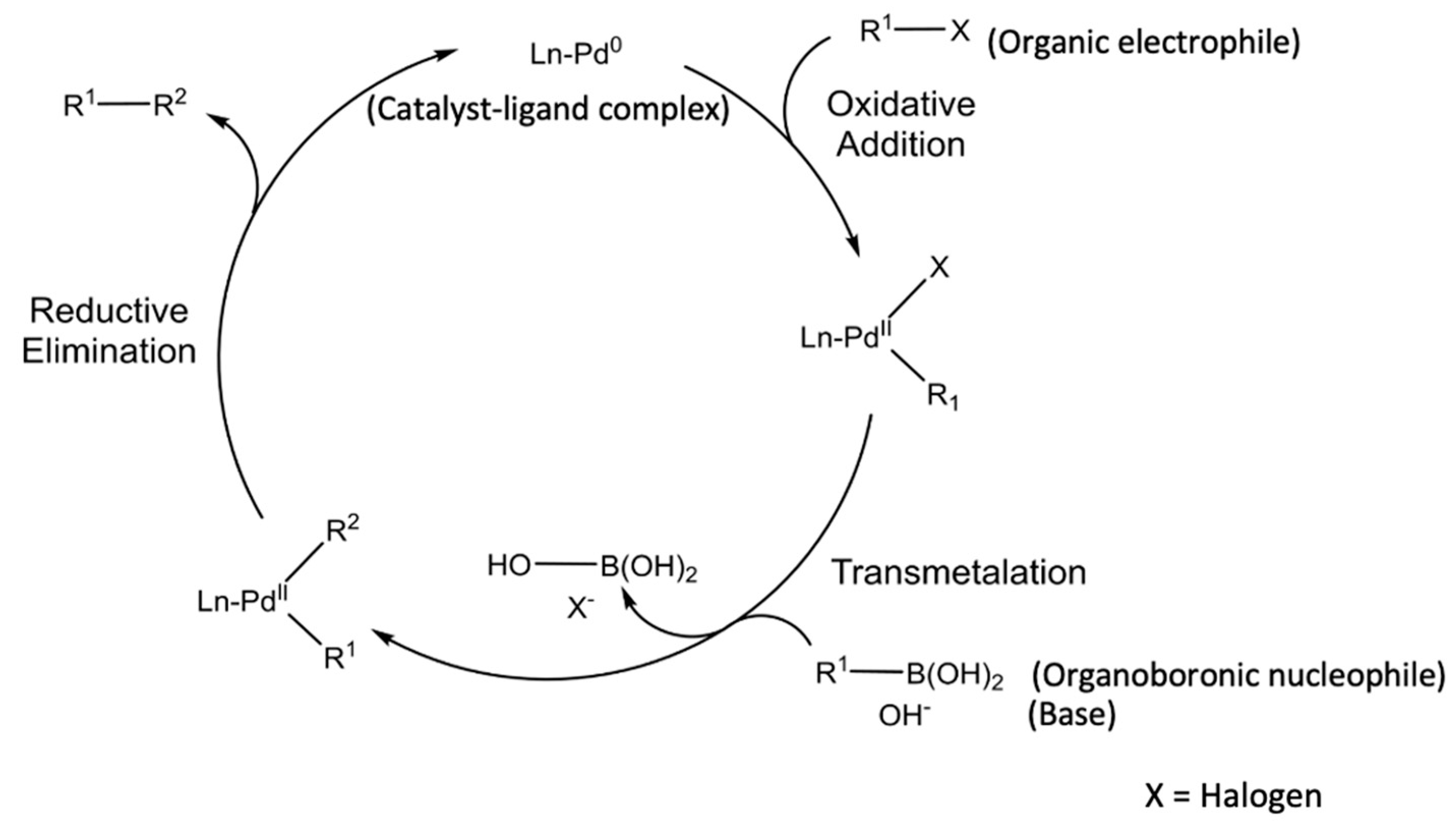
Knowledge | Free Full-Text | Catalyst Recycling in the Suzuki Coupling Reaction: Toward a Greener Synthesis in the Pharmaceutical Industry

Base-Free Suzuki–Miyaura Coupling Reaction Using Palladium(II) Supported Catalyst in Water | Catalysis Letters
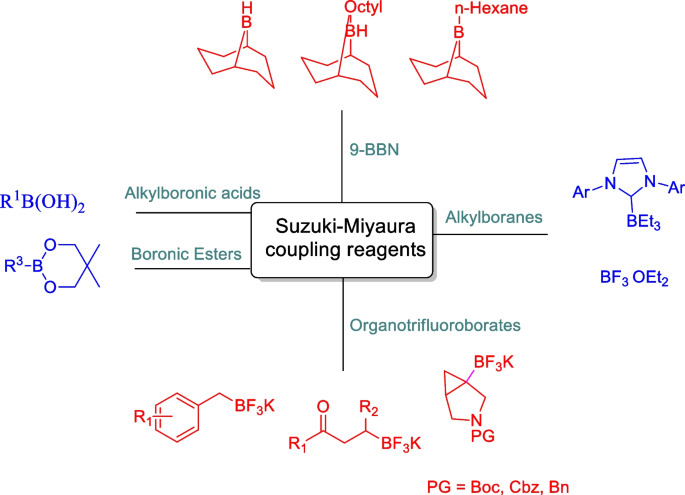
Suzuki–Miyaura cross-couplings for alkyl boron reagent: recent developments—a review | Future Journal of Pharmaceutical Sciences | Full Text